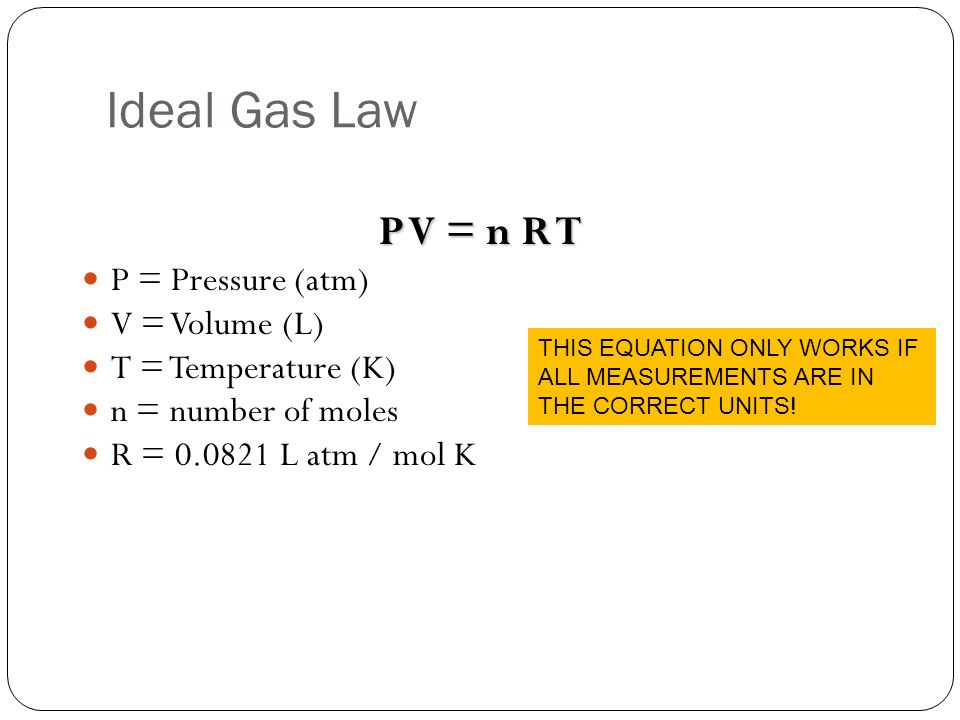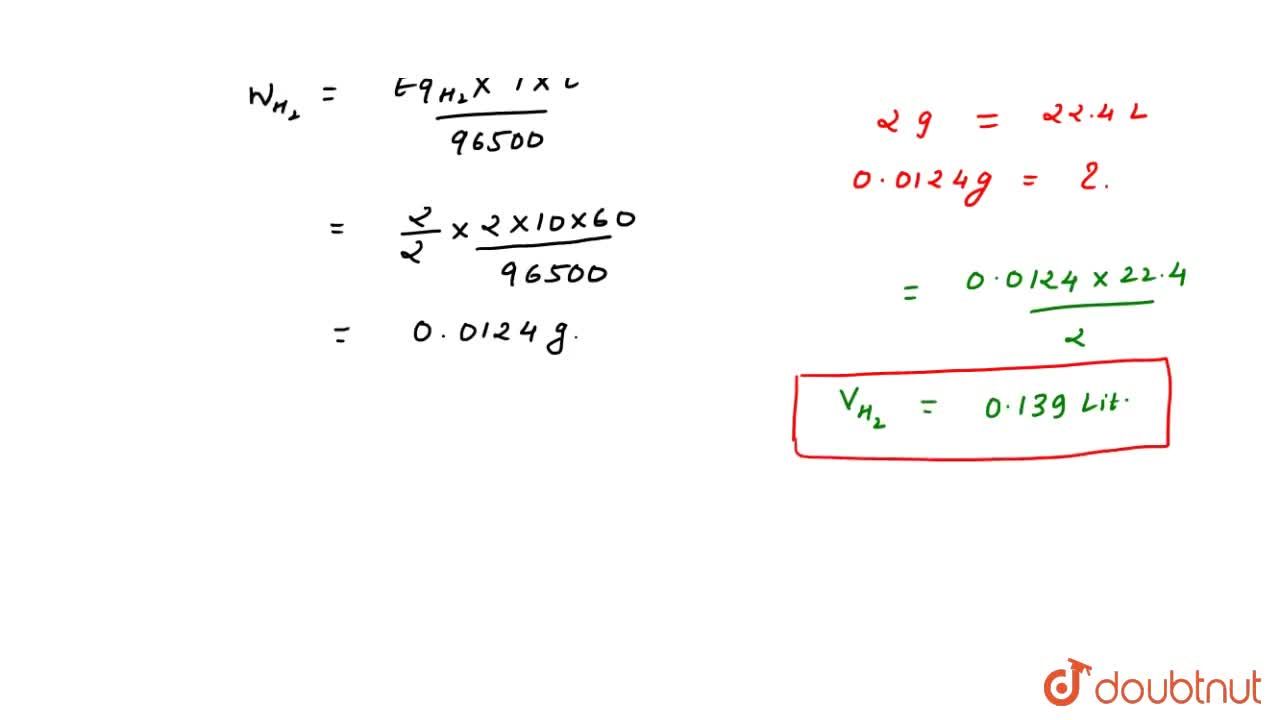
Calculate the volume of gases liberated at anode and cathode at NTP from the electrolysis of Na(2)SO(4)(aq.) solution by a current of 2 ampere passed for 10 minute.
Calculate the volume of gases liberated at anode and cathode at NTP from the electrolysis of Na2SO4(aq.) - Sarthaks eConnect | Largest Online Education Community
![High School:Stoichiometry] Calculating volume of gas. I got 400 but even my teacher only got 480. The answer is C. : r/chemistryhomework High School:Stoichiometry] Calculating volume of gas. I got 400 but even my teacher only got 480. The answer is C. : r/chemistryhomework](https://i.redd.it/j69a24fc57y31.jpg)
High School:Stoichiometry] Calculating volume of gas. I got 400 but even my teacher only got 480. The answer is C. : r/chemistryhomework

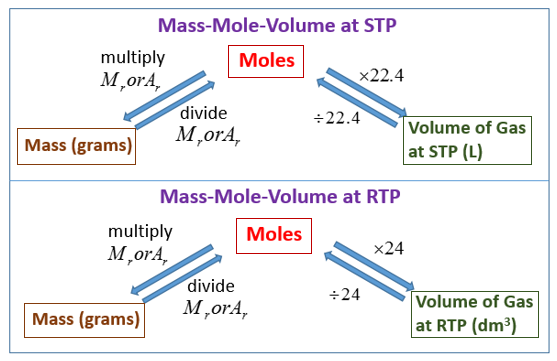
molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises